化工进展 ›› 2022, Vol. 41 ›› Issue (9): 4986-4994.DOI: 10.16085/j.issn.1000-6613.2021-2350
HNO3改性活性炭对不同价态离子的电吸附规律
- 1.西安工程大学环境与化学工程学院,陕西 西安 710048
2.中国电建集团北京勘测设计研究院有限公司,北京 100024
-
收稿日期:2021-11-16修回日期:2022-03-21出版日期:2022-09-25发布日期:2022-09-27 -
通讯作者:李海红 -
作者简介:袁权(1998—),男,硕士研究生,研究方向为电化学。E-mail:834878981@qq.com。 -
基金资助:陕西省科技厅社会发展领域项目(2020SF-435);榆林市科技计划(CXY-2020-054)
Electric adsorption laws of HNO3-modified activated carbon for different valence ions
YUAN Quan1( ), LI Haihong1(
), LI Haihong1( ), LIU Haojie2
), LIU Haojie2
- 1.College of Environmental and Chemical Engineering, Xi’an Polytechnic University, Xi’an 710048, Shaanxi, China
2.CEC Beijing Survey and Design Institute Co. , Ltd. , Beijing 100024, China
-
Received:2021-11-16Revised:2022-03-21Online:2022-09-25Published:2022-09-27 -
Contact:LI Haihong
摘要:
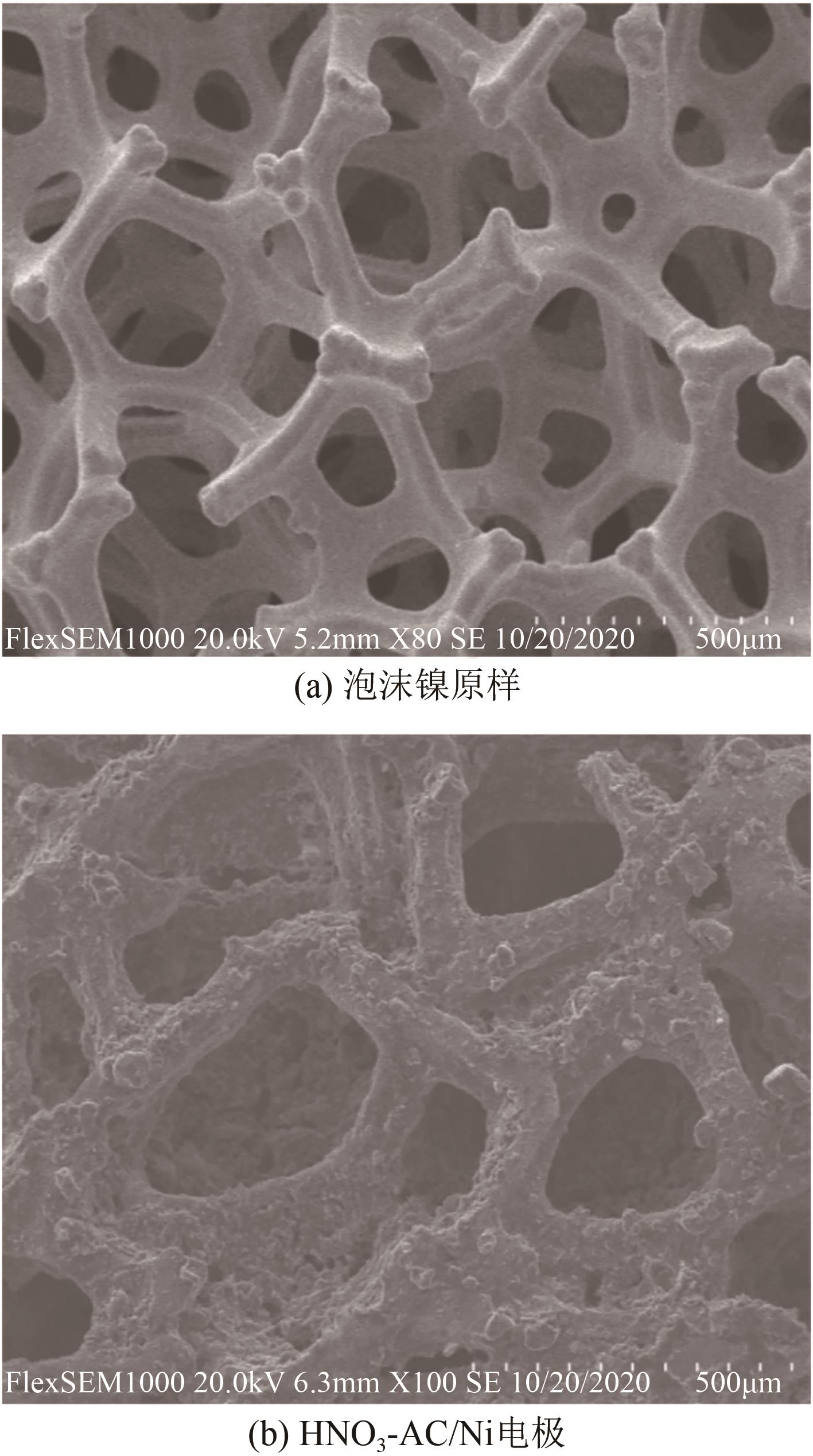
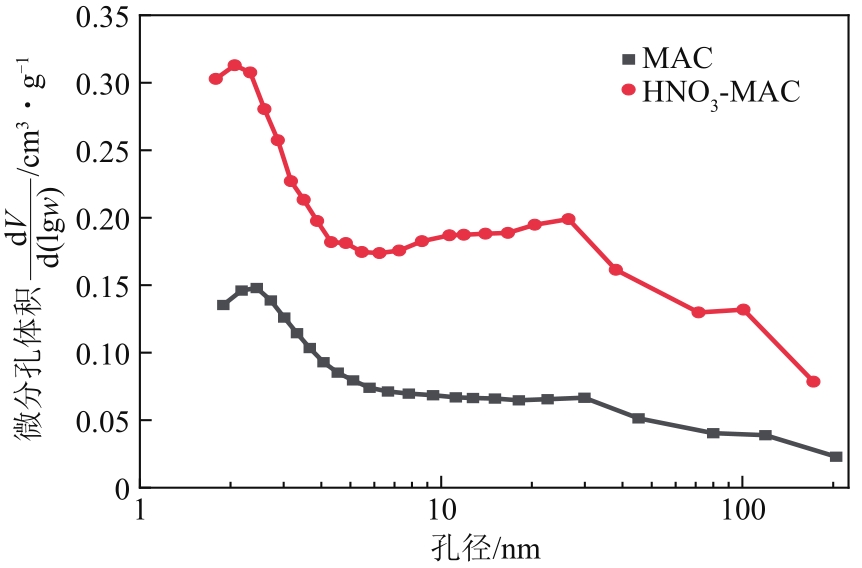
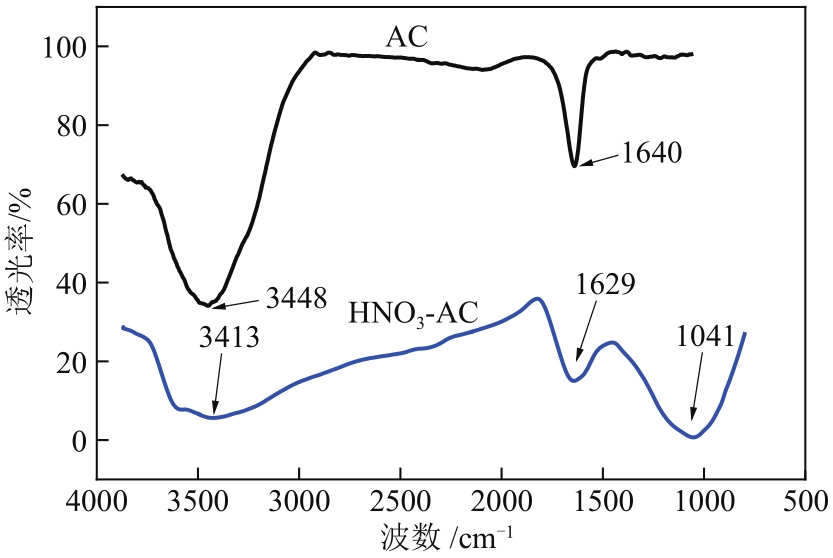
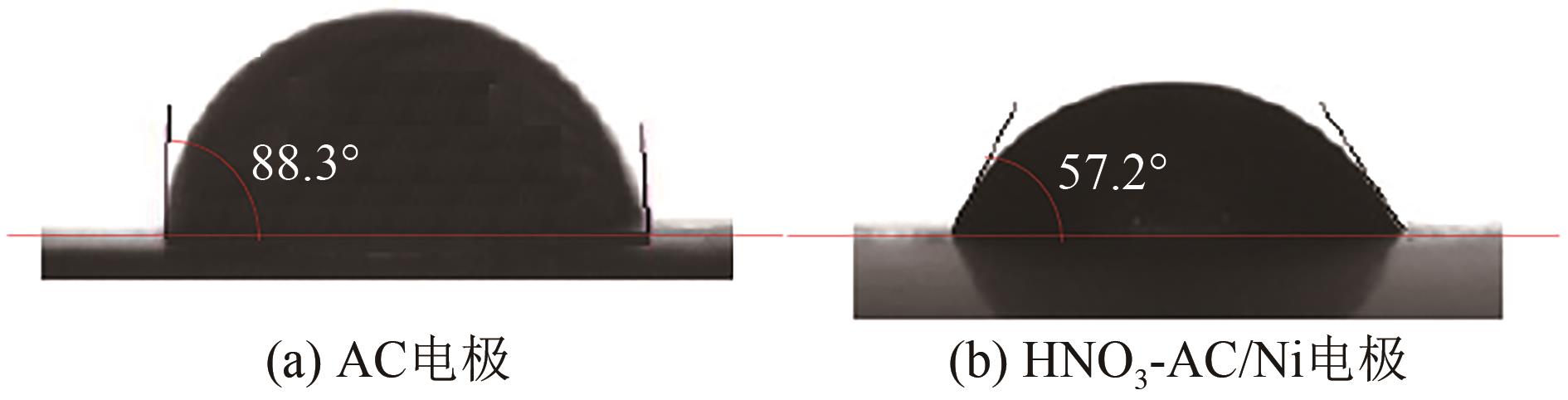
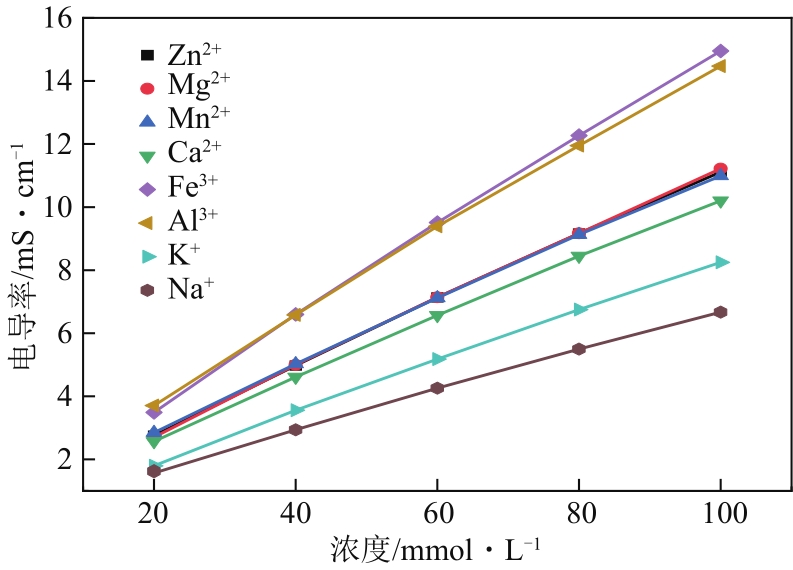
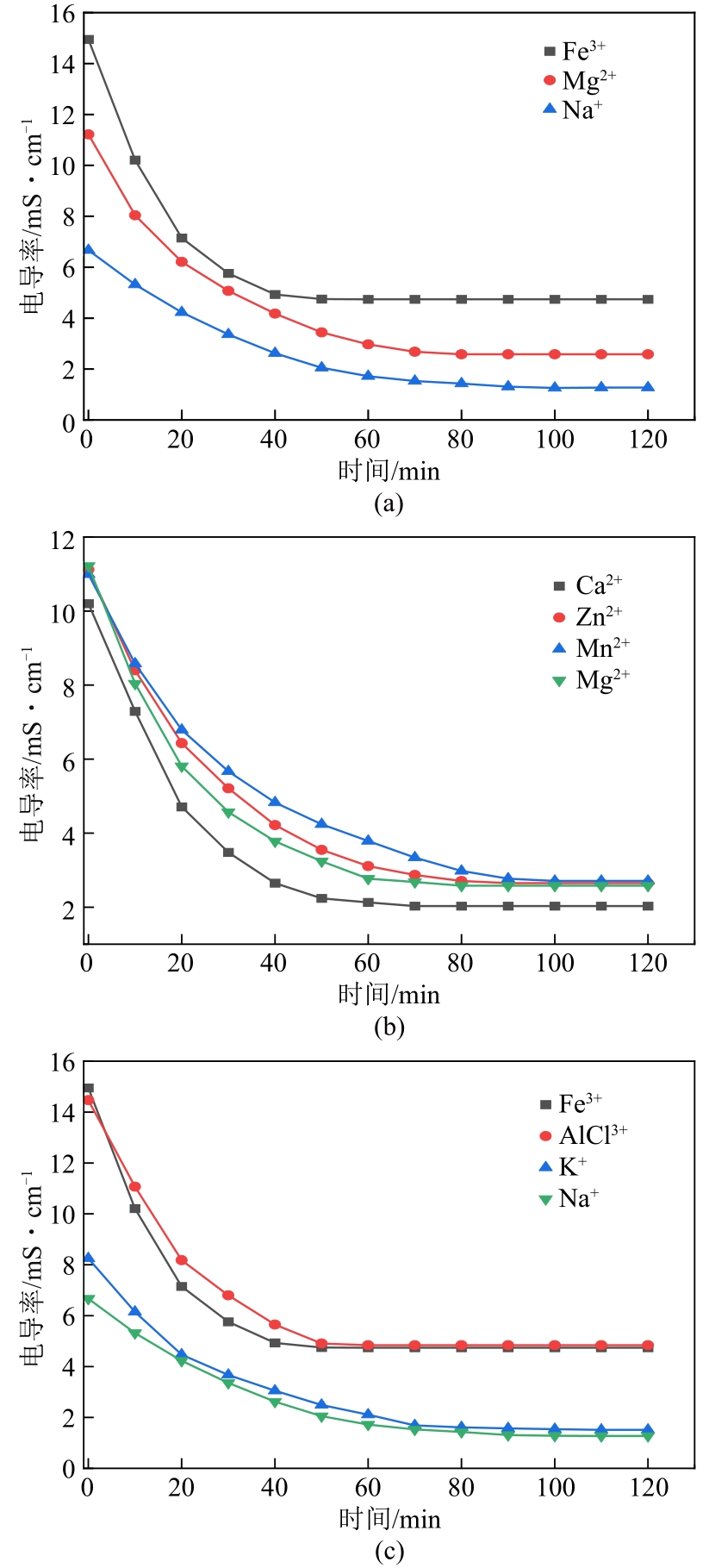
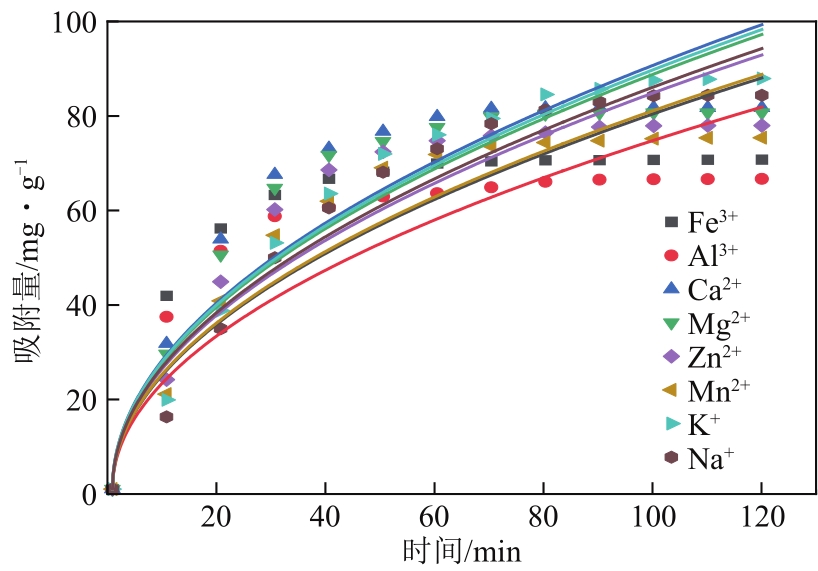
以硝酸改性活性炭为原材料,制备电吸附电极,并研究其对8种常见金属盐离子的吸附特性;分别采用扫描电镜、比表面积及孔径分析仪、红外光谱仪和电化学工作站等对改性前后材料的性能进行表征和分析。结果表明:改性后的活性炭相比于改性前拥有更好的孔隙结构,含氧官能团增多,制备出的电极电化学性能更好;在除盐实验中,制备的电极对价态越高的离子去除速率越快但去除率越低;对于同价态离子,水合离子半径越小时去除速率越快且去除率越高;离子从溶液到电极表面再到活性材料孔道内部的过程,主要为物理吸附过程,也存在较微弱的化学吸附。
中图分类号:
引用本文
袁权, 李海红, 刘浩杰. HNO3改性活性炭对不同价态离子的电吸附规律[J]. 化工进展, 2022, 41(9): 4986-4994.
YUAN Quan, LI Haihong, LIU Haojie. Electric adsorption laws of HNO3-modified activated carbon for different valence ions[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4986-4994.
使用本文
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| AC | 148.17 | 0.16 | 4.3 |
| HNO3-AC | 341.38 | 0.42 | 4.9 |
表1 改性前后AC的比表面积及孔结构参数
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| AC | 148.17 | 0.16 | 4.3 |
| HNO3-AC | 341.38 | 0.42 | 4.9 |
| 改性前后AC | C 1s | O 1s | N 1s |
|---|---|---|---|
| AC | 69.22 | 14.49 | 1.05 |
| HNO3-AC | 65.16 | 17.97 | 2.17 |
表2 改性前后AC表面化学成分 (%)
| 改性前后AC | C 1s | O 1s | N 1s |
|---|---|---|---|
| AC | 69.22 | 14.49 | 1.05 |
| HNO3-AC | 65.16 | 17.97 | 2.17 |
| 离子种类 | σ-c关系方程 | 相关系数R2 |
|---|---|---|
| Zn2+ | σ=0.1046c+0.757 | 0.9993 |
| Mg2+ | σ=0.1061c+0.683 | 0.9993 |
| Mn2+ | σ=0.1019c+0.91 | 0.9991 |
| Ca2+ | σ=0.0955c+0.75 | 0.9991 |
| Fe3+ | σ=0.143c+0.787 | 0.9991 |
| Al3+ | σ=0.1345c+1.153 | 0.9991 |
| K+ | σ=0.0805c+0.281 | 0.9991 |
| Na+ | σ=0.0638c+0.36 | 0.9990 |
表3 不同离子的标准曲线关系方程
| 离子种类 | σ-c关系方程 | 相关系数R2 |
|---|---|---|
| Zn2+ | σ=0.1046c+0.757 | 0.9993 |
| Mg2+ | σ=0.1061c+0.683 | 0.9993 |
| Mn2+ | σ=0.1019c+0.91 | 0.9991 |
| Ca2+ | σ=0.0955c+0.75 | 0.9991 |
| Fe3+ | σ=0.143c+0.787 | 0.9991 |
| Al3+ | σ=0.1345c+1.153 | 0.9991 |
| K+ | σ=0.0805c+0.281 | 0.9991 |
| Na+ | σ=0.0638c+0.36 | 0.9990 |
| 参数 | 一价离子 | 二价离子 | 三价离子 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mn2+ | Zn2+ | Mg2+ | Ca2+ | Al3+ | Fe3+ | |||
| 去除率/% | 80.86 | 81.7 | 75.34 | 76.17 | 77 | 80.1 | 66.55 | 68.29 | ||
| 吸附平衡时间/min | 100 | 95 | 90 | 85 | 80 | 70 | 55 | 50 | ||
| 水合离子半径/nm | 0.358 | 0.331 | 0.438 | 0.43 | 0.428 | 0.412 | 0.475 | 0.457 | ||
表4 各离子的去除率、吸附平衡时间及水合离子半径
| 参数 | 一价离子 | 二价离子 | 三价离子 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mn2+ | Zn2+ | Mg2+ | Ca2+ | Al3+ | Fe3+ | |||
| 去除率/% | 80.86 | 81.7 | 75.34 | 76.17 | 77 | 80.1 | 66.55 | 68.29 | ||
| 吸附平衡时间/min | 100 | 95 | 90 | 85 | 80 | 70 | 55 | 50 | ||
| 水合离子半径/nm | 0.358 | 0.331 | 0.438 | 0.43 | 0.428 | 0.412 | 0.475 | 0.457 | ||
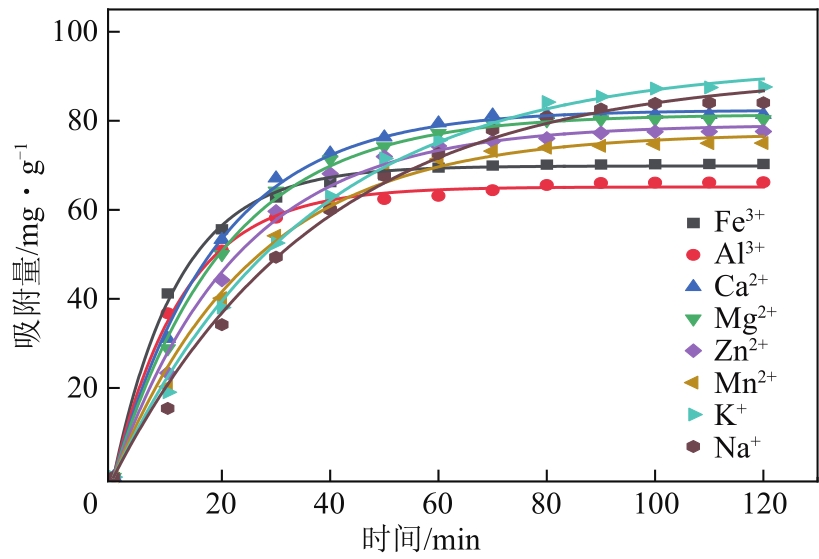
| 离子种类 | qe/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 69.84203 | 0.08277 | 0.99792 |
| Al3+ | 65.14445 | 0.07696 | 0.99598 |
| Ca2+ | 82.3935 | 0.05238 | 0.9981 |
| Mg2+ | 81.41524 | 0.04876 | 0.99792 |
| Zn2+ | 79.13729 | 0.04362 | 0.99351 |
| Mn2+ | 77.26577 | 0.03864 | 0.99389 |
| K+ | 92.71654 | 0.02779 | 0.99718 |
| Na+ | 90.71137 | 0.02605 | 0.99354 |
表5 不同离子的准一级吸附模型拟合参数
| 离子种类 | qe/mg·g-1 | k1/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 69.84203 | 0.08277 | 0.99792 |
| Al3+ | 65.14445 | 0.07696 | 0.99598 |
| Ca2+ | 82.3935 | 0.05238 | 0.9981 |
| Mg2+ | 81.41524 | 0.04876 | 0.99792 |
| Zn2+ | 79.13729 | 0.04362 | 0.99351 |
| Mn2+ | 77.26577 | 0.03864 | 0.99389 |
| K+ | 92.71654 | 0.02779 | 0.99718 |
| Na+ | 90.71137 | 0.02605 | 0.99354 |
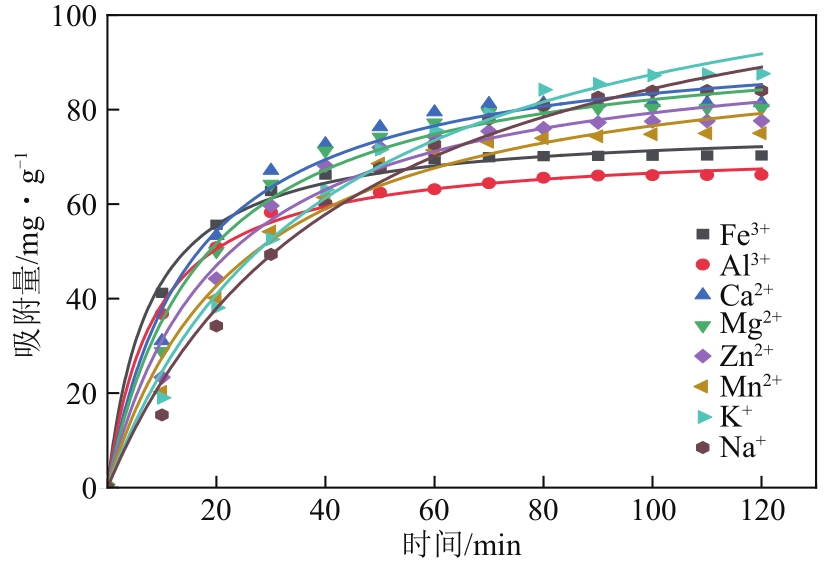
| 离子种类 | qe/mg·g-1 | k2/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 76.74263 | 0.00171 | 0.99503 |
| Al3+ | 72.30318 | 0.00160 | 0.99679 |
| Ca2+ | 96.3138 | 0.00007 | 0.98231 |
| Mg2+ | 96.30625 | 0.00060 | 0.98293 |
| Zn2+ | 95.72711 | 0.00050 | 0.97558 |
| Mn2+ | 95.52012 | 0.00042 | 0.97795 |
| K+ | 122.19321 | 0.00021 | 0.98011 |
| Na+ | 121.75304 | 0.00019 | 0.98467 |
表6 不同离子的准二级吸附模型拟合参数
| 离子种类 | qe/mg·g-1 | k2/min-1 | R2 |
|---|---|---|---|
| Fe3+ | 76.74263 | 0.00171 | 0.99503 |
| Al3+ | 72.30318 | 0.00160 | 0.99679 |
| Ca2+ | 96.3138 | 0.00007 | 0.98231 |
| Mg2+ | 96.30625 | 0.00060 | 0.98293 |
| Zn2+ | 95.72711 | 0.00050 | 0.97558 |
| Mn2+ | 95.52012 | 0.00042 | 0.97795 |
| K+ | 122.19321 | 0.00021 | 0.98011 |
| Na+ | 121.75304 | 0.00019 | 0.98467 |
| 离子种类 | kn /min-1 | R2 |
|---|---|---|
| Fe3+ | 8.01149 | 0.46896 |
| Al3+ | 7.44364 | 0.40818 |
| Ca2+ | 9.04892 | 0.41207 |
| Mg2+ | 8.8568 | 0.37921 |
| Zn2+ | 8.45852 | 0.34302 |
| Mn2+ | 8.07978 | 0.29454 |
| K+ | 8.95356 | 0.21725 |
| Na+ | 8.58248 | 0.22528 |
表7 不同离子的颗粒内扩散模型拟合参数
| 离子种类 | kn /min-1 | R2 |
|---|---|---|
| Fe3+ | 8.01149 | 0.46896 |
| Al3+ | 7.44364 | 0.40818 |
| Ca2+ | 9.04892 | 0.41207 |
| Mg2+ | 8.8568 | 0.37921 |
| Zn2+ | 8.45852 | 0.34302 |
| Mn2+ | 8.07978 | 0.29454 |
| K+ | 8.95356 | 0.21725 |
| Na+ | 8.58248 | 0.22528 |
| 1 | 马双忱, 刘畅, 马岚, 等. 电吸附用于微污染水处理: 技术选择、工艺原理、未来发展[J]. 化工进展, 2020, 39(7): 2841-2849. |
| MA Shuangchen, LIU Chang, MA Lan, et al. Electro-adsorption for micro-polluted water treatment: technology selection, process principle, future development[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2841-2849. | |
| 2 | MAHER M, HASSAN S, SHOUEIR K, et al. Activated carbon electrode with promising specific capacitance based on potassium bromide redox additive electrolyte for supercapacitor application[J]. Journal of Materials Research and Technology, 2021, 11: 1232-1244. |
| 3 | FOLARANMI G, BECHELANY M, SISTAT P, et al. Comparative investigation of activated carbon electrode and a novel activated carbon/graphene oxide composite electrode for an enhanced capacitive deionization[J]. Materials, 2020, 13(22): 5185. |
| 4 | ALENCHERRY T, A R N, GHOSH S, et al. Effect of increasing electrical conductivity and hydrophilicity on the electrosorption capacity of activated carbon electrodes for capacitive deionization[J]. Desalination, 2017, 415: 14-19. |
| 5 | CHEN Zhaolin, SONG Cunyi, SUN Xiaowei, et al. Kinetic and isotherm studies on the electrosorption of NaCl from aqueous solutions by activated carbon electrodes[J]. Desalination, 2011, 267(2/3): 239-243. |
| 6 | 骆青虎, 武福平, 李锡锋, 等. 碱改性活性炭纤维电吸附处理RO浓水效果及除盐动力学特性[J]. 环境工程学报, 2019, 13(11): 2545-2552. |
| LUO Qinghu, WU Fuping, LI Xifeng, et al. Electrosorption effect and desalination kinetic characteristics of reverse osmosis concentrated water with alkaline modified activated carbon fiber electrode[J]. Chinese Journal of Environmental Engineering, 2019, 13(11): 2545-2552. | |
| 7 | MORENO-CASTILLA C, CARRASCO-MARÍN F, MALDONADO-HÓDAR F J, et al. Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content[J]. Carbon, 1998, 36(1/2): 145-151. |
| 8 | 宾齐, 李海红, 张田田. 活化剂改性秸秆基活性炭的制备及其表征[J]. 纺织高校基础科学学报, 2020, 33(4): 111-117. |
| Qi BIN, LI Haihong, ZHANG Tiantian. Preparation and characterization of cotton stalk-based activated carbon modified by activator[J]. Basic Sciences Journal of Textile Universities, 2020, 33(4): 111-117. | |
| 9 | 李海红, 李红艳, 夏禹周. 活性炭涂层电极的制备及其电化学性能[J]. 材料科学与工程学报, 2014, 32(1): 101-106. |
| LI Haihong, LI Hongyan, XIA Yuzhou. Preparation and electrochemical performance of activated carbon coated electrodes[J]. Journal of Materials Science and Engineering, 2014, 32(1): 101-106. | |
| 10 | 李海红, 张超, 董军旗, 等. 活性炭负载TiO2改性处理及其性能表征[J]. 粉末冶金材料科学与工程, 2015, 20(3): 438-443. |
| LI Haihong, ZHANG Chao, DONG Junqi, et al. Preparation and characterization of active carbon material modified by TiO2 [J]. Materials Science and Engineering of Powder Metallurgy, 2015, 20(3): 438-443. | |
| 11 | 陈小娟, 张伟庆, 余小岚, 等. 适用于本科教学的BET比表面测定实验[J]. 大学化学, 2017, 32(7): 60-67. |
| CHEN Xiaojuan, ZHANG Weiqing, YU Xiaolan, et al. Measuring BET specific surface area: a new experiment for undergraduate teaching[J]. University Chemistry, 2017, 32(7): 60-67. | |
| 12 | CYCHOSZ STRUCKHOFF K, THOMMES M, SARKISOV L. On the universality of capillary condensation and adsorption hysteresis phenomena in ordered and crystalline mesoporous materials[J]. Advanced Materials Interfaces, 2020, 7(12): 2000184. |
| 13 | 王芳平, 马婧, 李小亚, 等. 板栗壳生物炭高性能对称性超级电容器电极材料的制备及性能[J]. 化工进展, 2021, 40(8): 4381-4387. |
| WANG Fangping, MA Jing, LI Xiaoya, et al. Preparation and properties of chestnut shell-based biochar electrode material for high-performance symmetrical supercapacitor[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4381-4387. | |
| 14 | 屈笑笑, 邢宝林, 康伟伟, 等. 玉米芯电容炭的制备及其电化学性能[J]. 化工进展, 2018, 37(6): 2340-2346. |
| QU Xiaoxiao, XING Baolin, KANG Weiwei, et al. Preparation and electrochemical performance of capacitive carbon derived from corncob[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2340-2346. | |
| 15 | 赵思宇, 李曜, 樊晗晗, 等. 蔗渣基活性炭孔结构的调控及其对超级电容器电化学性能的影响研究[J]. 中国造纸学报, 2021, 36(2): 39-49. |
| ZHAO Siyu, LI Yao, FAN Hanhan, et al. Regulation of the pore structure of bagasse-based activated carbon and its effect on the electrochemical properties of supercapacitor[J]. Transactions of China Pulp and Paper, 2021, 36(2): 39-49. | |
| 16 | SETIARSO P, SARI N P. Graphene oxide-paraffin-nanobentonite as working electrode for cyclic voltammetry analysis for nicotinic acid[J]. Asian Journal of Chemistry, 2021, 33(4): 757-761. |
| 17 | WADHAI S, JADHAV Y, THAKUR P. Synthesis of metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) composite: characterization, cyclic voltammetry and photocatalytic hydrogen evolution[J]. Solar Energy Materials and Solar Cells, 2021, 223: 110958. |
| 18 | KILIC A, EROGLU D. Characterization of the effect of cell design on Li-S battery resistance using electrochemical impedance spectroscopy[J]. ChemElectroChem, 2021, 8(5): 963-971. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [3] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [4] | 邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539. |
| [5] | 刘雅娟. 浸没式PAC-AMBRs系统中PAC缓解膜污染的研究进展[J]. 化工进展, 2023, 42(1): 457-468. |
| [6] | 孙宪航, 任铸, 张国军, 孙媛, 范开峰, 黄维秋. 超临界CO2作用下甲苯在活性炭中的脱附机理[J]. 化工进展, 2022, 41(S1): 631-636. |
| [7] | 刘楠, 胡一铭, 杨颖, 李红晋, 高竹青, 郝秀丽. 废旧聚丙烯/活性炭微波共裂解制取可燃裂解气与轻质裂解油[J]. 化工进展, 2022, 41(S1): 150-159. |
| [8] | 张辛亥, 赵思琛, 朱辉, 张首石, 王凯. 多种碳材料与碳酸钠复合后脱硫性能对比[J]. 化工进展, 2022, 41(S1): 424-435. |
| [9] | 何晨露, 邱晨茜, 方娟, 杨旋, 赖建军, 郑新宇, 吕建华, 陈燕丹, 黄彪. 基于低共熔溶剂体系的氮掺杂超级电容炭[J]. 化工进展, 2022, 41(9): 4946-4953. |
| [10] | 熊永志, 刘艳艳, 陈晓荭, 卢贝丽, 黄彪, 林冠烽. 甘蔗渣基磷掺杂活性炭的制备及其电化学性能[J]. 化工进展, 2022, 41(8): 4397-4405. |
| [11] | 黄平安, 徐俊, 杨宇轩, 潘宇涵, 王新文, 黄群星. 球磨改性热解炭吸附磺胺甲 唑[J]. 化工进展, 2022, 41(7): 3784-3793. 唑[J]. 化工进展, 2022, 41(7): 3784-3793. |
| [12] | 郑敏, 徐垒, 陈晨, 徐忠宁, 付明来. 稀硝酸催化还原工艺中Pd/AC制备条件优化[J]. 化工进展, 2022, 41(7): 3938-3946. |
| [13] | 蔡思超, 周静, 杜金泽, 李方舟, 李源森, 何林, 李鑫钢, 王成扬. 煤化工酚基精馏釜残资源化利用过程初步分析[J]. 化工进展, 2022, 41(6): 3360-3371. |
| [14] | 陈泳, 马妍楠, 徐成. 吸附电活性染料的活性炭电极电化学性能[J]. 化工进展, 2022, 41(5): 2537-2545. |
| [15] | 钱科, 邓苗, 乔志军, 方志梅, 屠建飞, 阮殿波. 环糊精基活性炭的制备及其电化学性能[J]. 化工进展, 2022, 41(4): 2000-2006. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 546
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 254
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||