Dry and steam reforming of biomass pyrolysis gas for rich hydrogen gas |
| |
| Affiliation: | 1. Laboratory for Catalysis Engineering, School of Chemical and Bimolecular Engineering, The University of Sydney, NSW 2006, Australia;2. Energy Research Institute, University of Leeds, Leeds LS2 9JT, UK;3. School of Environmental Science & Engineering, Kunming University of Science & Technology, Kunming 650038, PR China;4. School of Engineering, University of Hull, Hull HU6 7RX, UK;1. Residues and Resource Reclamation Centre (R3C), Nanyang Environment and Water Research Institute, Nanyang Technological University, 1 Cleantech Loop, Clean Tech One, 637141, Singapore;2. School of Civil and Environmental Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore;1. Thermal and Environmental Engineering Institute, School of Mechanical Engineering, Tongji University, 1239 Siping Road, Shanghai, 200092, China;2. School of Mechanical Engineering, Inner Mongolia University of Technology, 49 Aimin Road, Hohhot, 010051, China;3. Key Laboratory of Renewable Energy, Chinese Academy of Sciences (CAS), Guangzhou Institute of Energy Conversion, CAS, China;1. Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, China;2. CAS Key Laboratory of Renewable Energy, China;3. Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, China;4. Institute of Nano Science and Technology, University of Science and Technology of China, Suzhou 215123, China;5. College of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, PR China |
| |
| Abstract: | 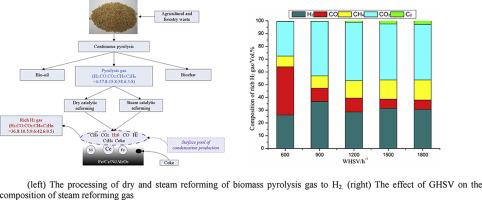
Biomass pyrolysis gas (including H2, CO, CH4, CO2, C2H4, C2H6 and etc.) reforming for hydrogen production over Ni/Fe/Ce/Al2O3 catalysts was presented in this study. This study investigated how the operating conditions, such as the calcinations temperature of catalysts, the reaction temperature, the gas hourly space velocity (GHSV) and the ratio of H2O/C, affect the conversion of CH4 and CO2 and the selectivity of hydrogen from dry and steam reforming of pyrolysis gas. The experimental results indicated that, under the conditions: the reaction temperature of 600 °C, the GHSV of 900 h−1 and H2O/C of 0.92, the reaction efficiency is the optimal. Especially, the concentration of H2, CO, CH4, CO2, and C2Hn (C2H4 and C2H6) were 36.80%, 10.48%, 9.61%, 42.62%, 0.49% respectively. The conversion of CH4 and CO2 reached 45.9% and 51.09%, respectively. There were all kinds of reactions during the processing of reforming of pyrolysis gas. And the main reactions changed with the operation condition. It was due to the promoting or inhibiting interaction among different constituents in the pyrolysis gas and the different activity of catalysts in the different operation condition. |
| |
| Keywords: | Pyrolysis gas Dry reforming Steam reforming Hydrogen Carbon deposition |
| 本文献已被 ScienceDirect 等数据库收录! |
|

