Adsorption of 2-phenylethyl alcohol on silica aerogel from saturated solution in supercritical CO2 |
| |
| Affiliation: | 1. Department of Chemical and Biochemical Engineering, College of Chemistry and Chemical Engineering, National Engineering Laboratory for Green Chemical Productions of Alcohols, Ethers and Esters, Xiamen University, Xiamen 361005, PR China;2. Technology Center of China Tobacco Fujian Industry Corporation, Xiamen 361022, PR China;1. Department of Biotechnology, I. M. Sechenov First Moscow State Medical University, 119991 Moscow, Russian Federation;2. Department of Technology of Chemico-Pharmaceutical and Cosmetic Substances, D. I. Mendeleev University of Chemical Technology of Russia, 125047 Moscow, Russian Federation;1. Research Department of Chemistry, Aditanar College of Arts and Science, Tiruchendur 628 216, Tamil Nadu, India;2. Department of Chemistry, Kamaraj College, Thoothukudi 628 003, Tamil Nadu, India |
| |
| Abstract: | 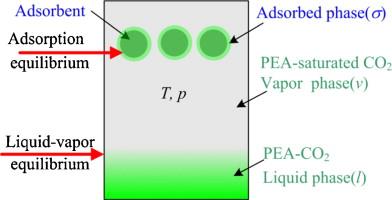
Adsorption of 2-phenylethyl alcohol (PEA) from supercritical CO2 onto silica aerogel was investigated. A monolayer to multilayer adsorption isotherm was observed, measured at 15.0 MPa and 323.2 K, from the PEA-unsaturated to PEA-saturated supercritical CO2, indicating the potential utility of the solute-saturated supercritical adsorption (SSA). The amount of PEA adsorbed on the silica aerogel with SSA at different temperatures and pressures was measured, and the release of PEA from the aerogel at 303.2 K was also evaluated. A theoretical model for the SSA equilibrium was developed with the assistance of the adsorption isotherms of pure CO2 onto the silica and considering a three-phase binary system, where the two-dimensional van der Waals equation of state and the three-dimensional Stryjek–Vera modification of the Peng–Robinson equation of state were used respectively to describe the adsorbed phase and the bulk phases (vapor phase and liquid phase). Results showed that the model was capable of describing the adsorption behavior of the system with an average absolute relative deviation of 3.3%. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

